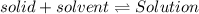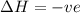Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

You know the right answer?
If the solution process is exothermic, then an increase in temperature usually results in:
Questions


Engineering, 10.12.2020 18:30



Mathematics, 10.12.2020 18:30



Advanced Placement (AP), 10.12.2020 18:30


Biology, 10.12.2020 18:30

Advanced Placement (AP), 10.12.2020 18:30

Physics, 10.12.2020 18:30

History, 10.12.2020 18:30

Social Studies, 10.12.2020 18:30

Mathematics, 10.12.2020 18:30




Mathematics, 10.12.2020 18:30

History, 10.12.2020 18:30