Identify which half-reactions occur at the anode
and cathode.
O2 + 2H2O + 4e + 40H-
Cd2...

Chemistry, 21.05.2021 01:00 ThatOneDumbAsian
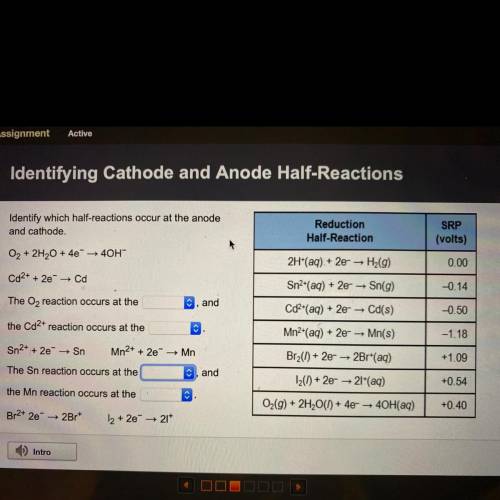
Identify which half-reactions occur at the anode
and cathode.
O2 + 2H2O + 4e + 40H-
Cd2+ + 2e → Cd
The O2 reaction occurs at the
C, and
the Cd2+ reaction occurs at the
Sn2+ + 2e → Sn
Mn2+ + 2e → Mn
The Sn reaction occurs at the
, and

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1
You know the right answer?
Questions

Biology, 29.07.2019 15:00

History, 29.07.2019 15:00





Mathematics, 29.07.2019 15:00

Mathematics, 29.07.2019 15:00

Computers and Technology, 29.07.2019 15:00



Health, 29.07.2019 15:00






Geography, 29.07.2019 15:00




