
Chemistry, 13.05.2021 20:10 sondrascott7351
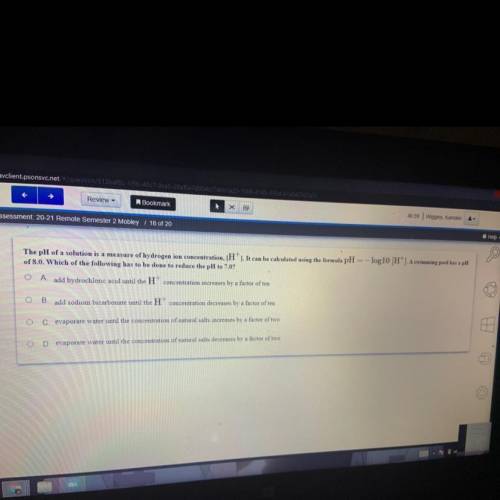
The pH of a solution is a measure of hydrogen ion concentration, (H'). It can be calculated using the formula pH = -log10 (H'). A swimming pool has a pH of 8.0. Which of the following has to be done to reduce the pH to 7.0?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
The pH of a solution is a measure of hydrogen ion concentration, (H'). It can be calculated using th...
Questions











Biology, 10.08.2019 01:20







Health, 10.08.2019 01:20


Biology, 10.08.2019 01:20



