
Chemistry, 11.05.2021 08:00 87haymaker
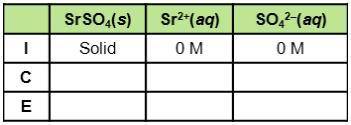
Consider the dissociation of SrSO4, which has a Ksp of 3.2 x 10-7. What do the three rows of (I, C,E) stand for in the table? How can the table be used to find equilibrium constants for this example?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2
You know the right answer?
Consider the dissociation of SrSO4, which has a Ksp of 3.2 x 10-7. What do the three rows of (I, C,E...
Questions

Social Studies, 11.02.2021 19:40

History, 11.02.2021 19:40




Biology, 11.02.2021 19:40

Health, 11.02.2021 19:40



Mathematics, 11.02.2021 19:40


Mathematics, 11.02.2021 19:40








Mathematics, 11.02.2021 19:40



