
Chemistry, 05.05.2021 23:20 jtbrown0093
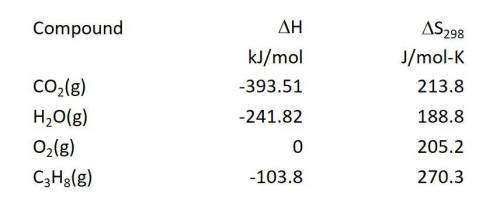
Calculate the entropy change in J/mol-K for the reaction: C3H8(g) + 5O2(g) ---> 3CO2(g) + 4H2O(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
Calculate the entropy change in J/mol-K for the reaction:
C3H8(g) + 5O2(g) ---> 3CO2(g) + 4H2O(...
Questions


Mathematics, 02.10.2020 16:01

Mathematics, 02.10.2020 16:01

Advanced Placement (AP), 02.10.2020 16:01



Mathematics, 02.10.2020 16:01

Spanish, 02.10.2020 16:01

Mathematics, 02.10.2020 16:01


History, 02.10.2020 16:01

Mathematics, 02.10.2020 16:01

History, 02.10.2020 16:01


Mathematics, 02.10.2020 16:01


Advanced Placement (AP), 02.10.2020 16:01

Mathematics, 02.10.2020 16:01




