Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

You know the right answer?
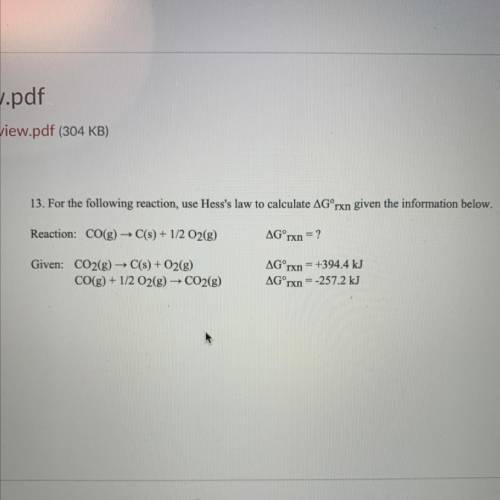
13. For the following reaction, use Hess's law to calculate AGʻrxn given the information below.
Re...
Questions

Computers and Technology, 28.03.2020 02:42





Physics, 28.03.2020 02:42




Mathematics, 28.03.2020 02:42

Mathematics, 28.03.2020 02:42








Geography, 28.03.2020 02:42