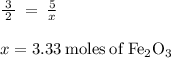Chemistry, 26.04.2021 21:30 roperbailey
Calculate the number of moles of iron (III) oxide (Fe 2 O 3 ) produced from 112 L of oxygen
(O 2 ) in the following reaction
4Fe(s) + 3O2 (g) = 2Fe2O3 (s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1


Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
Calculate the number of moles of iron (III) oxide (Fe 2 O 3 ) produced from 112 L of oxygen
(O 2 )...
Questions


English, 03.04.2020 02:12

History, 03.04.2020 02:12







Mathematics, 03.04.2020 02:12

Chemistry, 03.04.2020 02:12

History, 03.04.2020 02:12

Biology, 03.04.2020 02:12

Computers and Technology, 03.04.2020 02:12



Mathematics, 03.04.2020 02:12

Advanced Placement (AP), 03.04.2020 02:12


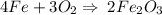

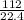 (Since one mole at STP = 22.4 L)
(Since one mole at STP = 22.4 L)