
Chemistry, 26.04.2021 21:10 tamikagoss22
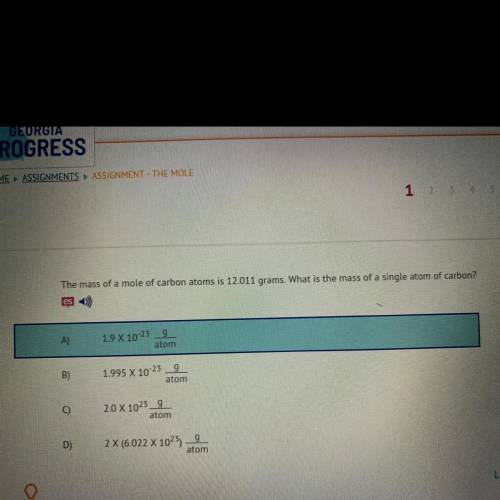
The mass of a mole of carbon atoms is 12.011 grams. What is the mass of a single atom of carbon?
A)9
1.9 X 10-23
atom
B)
g
1.995 X 10-23
atom
C)
2.0 X 1023_9
atom
D)
2 X (6.022 X 1025)
9
atom
Law of Conserv
Y

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3


Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2
You know the right answer?
The mass of a mole of carbon atoms is 12.011 grams. What is the mass of a single atom of carbon?
A...
Questions

Biology, 24.04.2020 22:55



Mathematics, 24.04.2020 22:55




Mathematics, 24.04.2020 22:55


Mathematics, 24.04.2020 22:55

Biology, 24.04.2020 22:55

Social Studies, 24.04.2020 22:55




Mathematics, 24.04.2020 22:55



Chemistry, 24.04.2020 22:55

Mathematics, 24.04.2020 22:55



