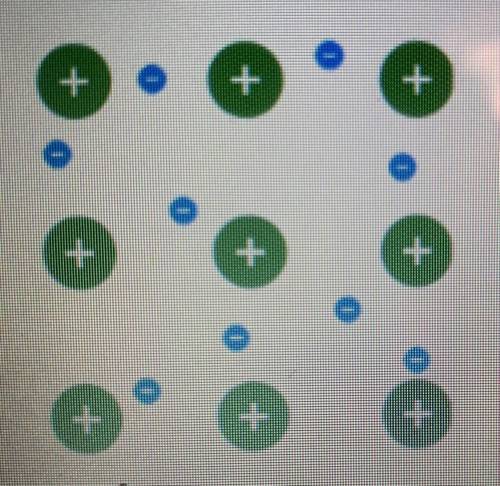
Examine the model of a metallic bond.
What do the smaller circles represent?
o the cations h...

Examine the model of a metallic bond.
What do the smaller circles represent?
o the cations held in place by the attractive forces within the sea of electrons
O the delocalized electrons that are free to move around the positive cations
the distinct and rigid bond between the different metal atoms
the unshared pairs of electrons of the metal atoms involved in the metallic bond

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Questions




English, 12.07.2019 15:10



Mathematics, 12.07.2019 15:10

Social Studies, 12.07.2019 15:10

Social Studies, 12.07.2019 15:10

Chemistry, 12.07.2019 15:10

Business, 12.07.2019 15:10

Biology, 12.07.2019 15:10

Geography, 12.07.2019 15:10

Biology, 12.07.2019 15:10


Chemistry, 12.07.2019 15:10


History, 12.07.2019 15:10




