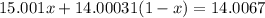Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
:nitrogen has two isotopes, n-14 and n-15, with atomic masses of 14.00031 amu and 15.001 amu, respec...
Questions


English, 05.11.2020 19:10



Computers and Technology, 05.11.2020 19:10

Mathematics, 05.11.2020 19:10

Mathematics, 05.11.2020 19:10

Computers and Technology, 05.11.2020 19:10

Mathematics, 05.11.2020 19:10

Mathematics, 05.11.2020 19:10


English, 05.11.2020 19:10

English, 05.11.2020 19:10

History, 05.11.2020 19:10

History, 05.11.2020 19:10



English, 05.11.2020 19:10

Mathematics, 05.11.2020 19:10