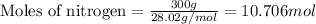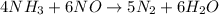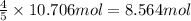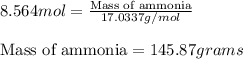Chemistry, 14.10.2019 21:40 WindelCaceus123
In the following reaction, how many grams of ammonia (nh3) will produce 300 grams of n2? 4nh3 + 6no → 5n2 + 6h2o the molar mass of ammonia is 17.0337 grams and that of nitrogen is 28.02 grams.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
In the following reaction, how many grams of ammonia (nh3) will produce 300 grams of n2? 4nh3 + 6no...
Questions

Mathematics, 30.04.2021 05:00



Mathematics, 30.04.2021 05:00

Mathematics, 30.04.2021 05:00

Advanced Placement (AP), 30.04.2021 05:00





Spanish, 30.04.2021 05:00

Physics, 30.04.2021 05:00


French, 30.04.2021 05:00

Physics, 30.04.2021 05:00


Chemistry, 30.04.2021 05:00



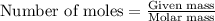 ....(1)
....(1)