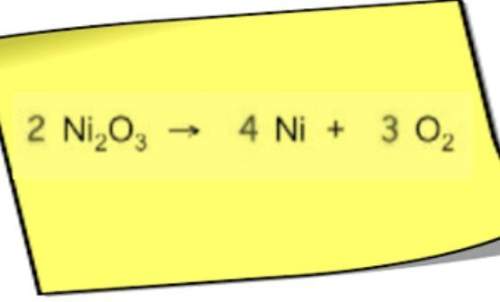
Consider the reaction below. c2h4(g) + h2(g) to c2h6(g) which change would likely cause the greatest increase in the rate of the reaction?
a decrease temperature and decrease pressure
b increase temperature and decrease pressure
c decrease temperature and increase pressure
d increase temperature and increase pressure

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
Consider the reaction below. c2h4(g) + h2(g) to c2h6(g) which change would likely cause the greatest...
Questions



Mathematics, 26.03.2021 19:20


Mathematics, 26.03.2021 19:20


Mathematics, 26.03.2021 19:20


Mathematics, 26.03.2021 19:20

Social Studies, 26.03.2021 19:20



Mathematics, 26.03.2021 19:20

Mathematics, 26.03.2021 19:20


Mathematics, 26.03.2021 19:20

History, 26.03.2021 19:20



History, 26.03.2021 19:20

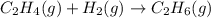 greatest increase in rate of reaction only occur when we increase the temperature and also increase the pressure.
greatest increase in rate of reaction only occur when we increase the temperature and also increase the pressure.