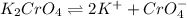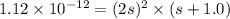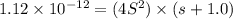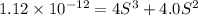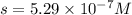Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Silver chromate is sparingly soluble in aqueous solutions. the ksp of ag2cro4 is 1.12× 10–12. what i...
Questions

Mathematics, 16.02.2021 21:00

Mathematics, 16.02.2021 21:00

Mathematics, 16.02.2021 21:00

Mathematics, 16.02.2021 21:00



Mathematics, 16.02.2021 21:00

Mathematics, 16.02.2021 21:00


Social Studies, 16.02.2021 21:00



English, 16.02.2021 21:00

Biology, 16.02.2021 21:00

Chemistry, 16.02.2021 21:00

Physics, 16.02.2021 21:00

English, 16.02.2021 21:00

Business, 16.02.2021 21:00

Mathematics, 16.02.2021 21:00

Mathematics, 16.02.2021 21:00

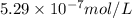
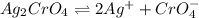
![K_{sp}=[Ag^{+}]^2[CrO_4^{-}]](/tpl/images/0248/2494/607c1.png)
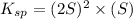
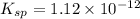
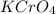 is written as:
is written as: