
Chemistry, 23.09.2019 05:30 montgomerykarloxc24x
Neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic mass of 40.0 g/mol. how would the number of atoms in a 1.0 mol sample of neon compare to the number of atoms in a 4.0 mol sample of argon

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

You know the right answer?
Neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic mass of 40.0 g/mo...
Questions

Mathematics, 17.01.2020 23:31

Mathematics, 17.01.2020 23:31

History, 17.01.2020 23:31




Arts, 17.01.2020 23:31

History, 17.01.2020 23:31


Mathematics, 17.01.2020 23:31


Physics, 17.01.2020 23:31


Mathematics, 17.01.2020 23:31


Mathematics, 17.01.2020 23:31

Biology, 17.01.2020 23:31

Biology, 17.01.2020 23:31

Geography, 17.01.2020 23:31

Mathematics, 17.01.2020 23:31

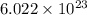 number of atoms.
number of atoms.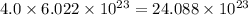 number of atoms
number of atoms

