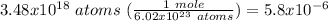Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Which equivalence factor should you use to convert from 3.48 x 1018 atoms of mg to moles of mg?
Questions

Mathematics, 10.02.2021 18:30

Mathematics, 10.02.2021 18:30

Mathematics, 10.02.2021 18:30

Mathematics, 10.02.2021 18:30

Computers and Technology, 10.02.2021 18:30

Arts, 10.02.2021 18:30



Mathematics, 10.02.2021 18:30


Biology, 10.02.2021 18:30

Biology, 10.02.2021 18:30


Mathematics, 10.02.2021 18:30



Chemistry, 10.02.2021 18:30