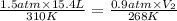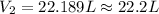Chemistry, 10.12.2019 04:31 Smartpotato9555
Agas has an initial volume of 15.4 l at a pressure of 1.5 atm and a temperature of 310 k. the pressure of the gas decreases to 0.9 atm as the temperature decreases to 268 k. what is the final volume of the gas? round your answer to the nearest tenth.
22.2 l

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Agas has an initial volume of 15.4 l at a pressure of 1.5 atm and a temperature of 310 k. the pressu...
Questions




















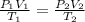
 = initial pressure of gas = 1.5 atm
= initial pressure of gas = 1.5 atm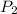 = final pressure of gas = 0.9 atm
= final pressure of gas = 0.9 atm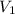 = initial volume of gas = 15.4 L
= initial volume of gas = 15.4 L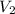 = final volume of gas = ?
= final volume of gas = ?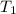 = initial temperature of gas = 310 K
= initial temperature of gas = 310 K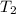 = final temperature of gas = 268 K
= final temperature of gas = 268 K