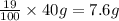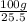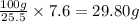A40.-g sample of impure kclo3 (solubility = 7.1 g per 100 g h2o at 20° c) is contaminated with 19 percent of kcl (solubility = 25.5 g per 100 g of h2o at 20° c). calculate the minimum quantity of 20° c water needed to dissolve all the kcl from the sample. (assume that the solubilities are unaffected by the presence of the other compound.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
A40.-g sample of impure kclo3 (solubility = 7.1 g per 100 g h2o at 20° c) is contaminated with 19 pe...
Questions

Biology, 04.12.2019 04:31



Mathematics, 04.12.2019 04:31

Social Studies, 04.12.2019 04:31




History, 04.12.2019 04:31

Business, 04.12.2019 04:31

Mathematics, 04.12.2019 04:31

Biology, 04.12.2019 04:31



History, 04.12.2019 04:31



History, 04.12.2019 04:31

Health, 04.12.2019 04:31

Social Studies, 04.12.2019 04:31

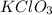 = 40 g
= 40 g