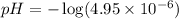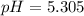Chemistry, 16.10.2019 12:00 weridness80
Determine the ph of a 0.05 m solution of hydrocyanic acid (hcn). hydrocyanic acid has an acid-dissociation constant of 4.9 × 10-10.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 23.06.2019 12:30
Atriple covalent bond involves two atoms sharing three pairs of electrons. true false
Answers: 2


Chemistry, 23.06.2019 16:00
Challenge question: this question is worth 6 points. as you saw in problem 9 we can have species bound to a central metal ion. these species are called ligands. in the past we have assumed all the d orbitals in some species are degenerate; however, they often are not. sometimes the ligands bound to a central metal cation can split the d orbitals. that is, some of the d orbitals will be at a lower energy state than others. ligands that have the ability to cause this splitting are called strong field ligands, cnâ’ is an example of these. if this splitting in the d orbitals is great enough electrons will fill low lying orbitals, pairing with other electrons in a given orbital, before filling higher energy orbitals. in question 7 we had fe2+, furthermore we found that there were a certain number (non-zero) of unpaired electrons. consider now fe(cn)6 4â’: here we also have fe2+, but in this case all the electrons are paired, yielding a diamagnetic species. how can you explain this?
Answers: 2
You know the right answer?
Determine the ph of a 0.05 m solution of hydrocyanic acid (hcn). hydrocyanic acid has an acid-dissoc...
Questions


Mathematics, 22.04.2020 01:54











English, 22.04.2020 01:54




Mathematics, 22.04.2020 01:54



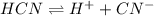
![k_a=\frac{[H^+][CN^-]}{[HCN]}](/tpl/images/0324/9828/15aab.png)
![[H^+]](/tpl/images/0324/9828/07acb.png) and
and 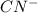 will be, 'x'
will be, 'x'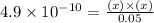
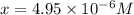
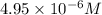
![pH=-\log [H^+]](/tpl/images/0324/9828/37e81.png)