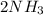Chemistry, 30.09.2019 00:30 markaylarowland8
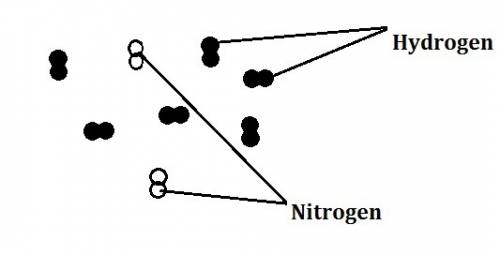
In the haber process, nitrogen (n2) and hydrogen (h2) are directly combined to form ammonia (nh3). which illustration contains the stoichiometric quantities of the reactants for this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
In the haber process, nitrogen (n2) and hydrogen (h2) are directly combined to form ammonia (nh3). w...
Questions


Physics, 28.06.2019 06:00






Mathematics, 28.06.2019 06:00





Social Studies, 28.06.2019 06:00

English, 28.06.2019 06:00




Mathematics, 28.06.2019 06:00

Mathematics, 28.06.2019 06:00

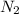 +
+ 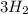 =
=