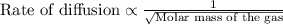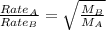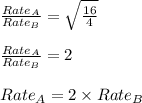Chemistry, 13.11.2019 13:31 zaniyastubbs9
Asample of gas a has a molar mass of 4 grams while a sample of gas b has a molar mass of 16 grams. which statement holds true?
both gas a and gas b diffuse at the same speed.
gas a effuses faster than gas b.
gas b effuses faster than gas a.
the molar masses of gas a and gas b are not related to effusion.
the molar mass is directly proportional to the rate of effusion.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
Asample of gas a has a molar mass of 4 grams while a sample of gas b has a molar mass of 16 grams. w...
Questions






Mathematics, 23.03.2020 20:26

Mathematics, 23.03.2020 20:26


Social Studies, 23.03.2020 20:26






Biology, 23.03.2020 20:26