
Chemistry, 23.03.2020 20:26 kcutler8603
Stearic acid, fatty acid commonly found in animal fat has a condensed Chemical formula CH3(CH2)16COOH. It is a common component of soaps and detergents. Which of the following statements is false?
A The -COOH group would experience hydrogen bonding with water
B The long alkane ''tail" CH3(CH2)16 -, is hydrophbic
C Stearic acid and water are probably miscible
D Stearic acid would probably dissolve in non polar solvents such as hexane C6H14
E Stearic acid contains hydrophobic regions

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
Stearic acid, fatty acid commonly found in animal fat has a condensed Chemical formula CH3(CH2)16COO...
Questions


English, 08.10.2021 20:00

Business, 08.10.2021 20:00


English, 08.10.2021 20:00

Mathematics, 08.10.2021 20:00

Mathematics, 08.10.2021 20:00





Mathematics, 08.10.2021 20:00






Biology, 08.10.2021 20:00

Mathematics, 08.10.2021 20:00

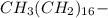 , is hydrophobic.
, is hydrophobic.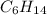 .
.

