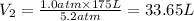Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
To what volume must 175 l of air at 1.0 atm be compressed to yield a pressure of 5.2 atm? (you can...
Questions


Mathematics, 15.08.2021 15:20

Physics, 15.08.2021 15:20




Physics, 15.08.2021 15:20

Physics, 15.08.2021 15:20


Mathematics, 15.08.2021 15:20



Mathematics, 15.08.2021 15:20

Mathematics, 15.08.2021 15:20


Mathematics, 15.08.2021 16:00

English, 15.08.2021 16:00

English, 15.08.2021 16:00


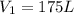
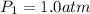
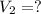
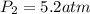
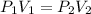 (constant temperature)
(constant temperature)