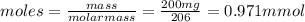Chemistry, 04.02.2020 12:58 savannahvargas512
Asap
1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers. each tablet contains 200 mg of ibuprofen, and a typical adult dose is two tablets every six hours.
• determine the molar mass of ibuprofen. show all steps to find the answer.
• calculate the number of moles of ibuprofen in a single tablet. show all steps to find the answer.
• calculate the number of moles of ibuprofen that an adult would have taken if she took four doses of ibuprofen in one day. show all steps to find the answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Asap
1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers....
1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers....
Questions



Advanced Placement (AP), 12.03.2021 04:10

Arts, 12.03.2021 04:10

English, 12.03.2021 04:10


Mathematics, 12.03.2021 04:10






Advanced Placement (AP), 12.03.2021 04:10

Mathematics, 12.03.2021 04:10

Mathematics, 12.03.2021 04:10


Mathematics, 12.03.2021 04:10

Mathematics, 12.03.2021 04:10

Mathematics, 12.03.2021 04:10

World Languages, 12.03.2021 04:10