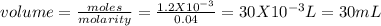Chemistry, 04.02.2020 12:58 abhibhambhani
20.0 ml of 0.06 m hcl (in a flask) is titrated with 0.04 m naoh (in a burette). how many milliliters of naoh needs to be used to reach the equivalence point?
a.0.03 ml
b.20.0 ml
c.20 ml
d.30 ml
2.which of the following would you identify a titration curve that involved a strong acid titrated by a weak base?
a. the ph at the equivalence point is lower than 7.
b. the ph at the equivalence point is higher than 7.
c. the titration curve begins at a higher ph and ends at a lower ph.
d. there is a rapid change in ph near the equivalence point (ph = 7).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
20.0 ml of 0.06 m hcl (in a flask) is titrated with 0.04 m naoh (in a burette). how many milliliters...
Questions

English, 25.08.2019 00:30






Mathematics, 25.08.2019 00:30

English, 25.08.2019 00:30


History, 25.08.2019 00:30

Health, 25.08.2019 00:30

Physics, 25.08.2019 00:30


Mathematics, 25.08.2019 00:30

Physics, 25.08.2019 00:30



Biology, 25.08.2019 00:30


History, 25.08.2019 00:30