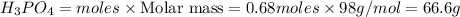Chemistry, 19.04.2021 07:50 Jlynbodmer
P4O10 + 6H20 → 4H3PO4 If 30 g of H2O reacts with 40g of P4O10, what is the limiting reactant? How much H3PO4 can be formed?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3


Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
P4O10 + 6H20 → 4H3PO4
If 30 g of H2O reacts with 40g of P4O10, what is the limiting reactant? How m...
Questions

English, 20.04.2021 04:40

Computers and Technology, 20.04.2021 04:40



Mathematics, 20.04.2021 04:50

Mathematics, 20.04.2021 04:50


Mathematics, 20.04.2021 04:50


Spanish, 20.04.2021 04:50


Mathematics, 20.04.2021 04:50



English, 20.04.2021 04:50



Mathematics, 20.04.2021 04:50


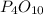 is the limiting reagent
is the limiting reagent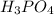 will be formed.
will be formed.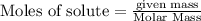
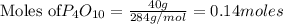
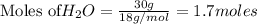
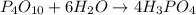
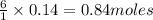 of
of 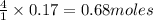 of
of