5.
Consider the equation for a saturated solution of potassium chromate:
K, CrO4(s)+ energy 5...

Chemistry, 12.04.2021 14:00 Knownothing
5.
Consider the equation for a saturated solution of potassium chromate:
K, CrO4(s)+ energy 5 2K+ (aq) + Cro. (aq)
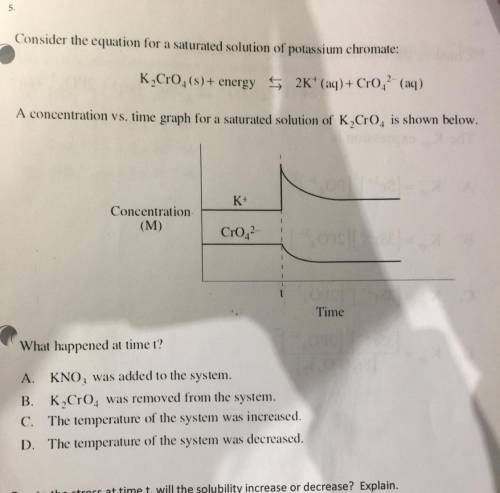
A concentration vs. time graph for a saturated solution of K Cr0is shown below.
K+
Concentration
(M)
Cr02
Time
What happened at time t?
A. KNO, was added to the system.
B. K Cro. was removed from the system.
C. The temperature of the system was increased.
D. The temperature of the system was decreased.
Due to the stress at time t, will the solubility increase or decrease? Explain.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1



Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Questions






Mathematics, 08.03.2020 19:00


History, 08.03.2020 19:02

Mathematics, 08.03.2020 19:02



Mathematics, 08.03.2020 19:03



Social Studies, 08.03.2020 19:04

Health, 08.03.2020 19:04


Mathematics, 08.03.2020 19:04

Geography, 08.03.2020 19:05




