
Chemistry, 06.04.2021 18:30 chewygamerz
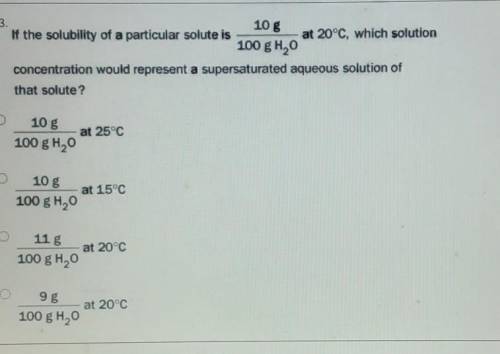
If the solubility of a particular solute is 10 g 100 g H2O at 20°C, which solution concentration would represent a supersaturated aqueous solution of that solute?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1


You know the right answer?
If the solubility of a particular solute is 10 g 100 g H2O at 20°C, which solution concentration wou...
Questions



Advanced Placement (AP), 18.03.2020 21:58


Mathematics, 18.03.2020 21:58








Mathematics, 18.03.2020 21:58


Social Studies, 18.03.2020 21:58

Mathematics, 18.03.2020 21:58






