Chemistry, 18.03.2020 21:58 emily965692
Manganese commonly occurs in nature as a mineral. The extraction of manganese from the carbonite mineral rhodochrosite, involves a two-step process. In the first step, manganese (II) carbonate and oxygen react to form manganese (IV) oxide and carbon dioxide: 2MnCO3(s)+O2(g)→2MnO2(s)+2CO2(g) In the second step, manganese (IV) oxide and aluminum react to form manganese and aluminum oxide: 3MnO2(s)+4Al(s)→3Mn(s)+2Al2O3(s) Write the net chemical equation for the production of manganese from manganese (II) carbonate, oxygen and aluminum. Be sure your equation is balanced.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
You know the right answer?
Manganese commonly occurs in nature as a mineral. The extraction of manganese from the carbonite min...
Questions


Mathematics, 07.10.2021 14:30


Biology, 07.10.2021 14:30

Mathematics, 07.10.2021 14:30

Mathematics, 07.10.2021 14:30

Chemistry, 07.10.2021 14:30

English, 07.10.2021 14:30

Social Studies, 07.10.2021 14:30

Social Studies, 07.10.2021 14:30




Social Studies, 07.10.2021 14:30



Biology, 07.10.2021 14:30

Social Studies, 07.10.2021 14:30

Social Studies, 07.10.2021 14:30

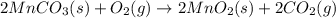 ( × 3)
( × 3)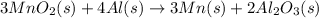 ( × 2)
( × 2)