
Chemistry, 02.04.2021 08:10 smithmorgan773p35885
If 10.00 moles of copper are reacted with 6.00 moles of sulfur according to the following balanced equation, which reactant is the limiter and how many moles of excess reactant would remain after the reaction is completed? 2 Cu + S --> Cu2S
Cu is limiting and 1.00 mole of excess S remain
S is limiting and 1.00 moles of excess Cu remain
S is limiting and 4.00 moles of excess Cu remain
Cu is limiting and 4.00 moles of excess S remain

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1


Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
You know the right answer?
If 10.00 moles of copper are reacted with 6.00 moles of sulfur according to the following balanced e...
Questions


Mathematics, 10.11.2020 21:20






Advanced Placement (AP), 10.11.2020 21:20

Mathematics, 10.11.2020 21:20


Geography, 10.11.2020 21:20


English, 10.11.2020 21:20

English, 10.11.2020 21:20



Spanish, 10.11.2020 21:20

Mathematics, 10.11.2020 21:20

Biology, 10.11.2020 21:20

Business, 10.11.2020 21:20

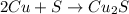
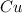 require 1 mole of
require 1 mole of 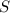
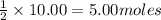 of
of 

