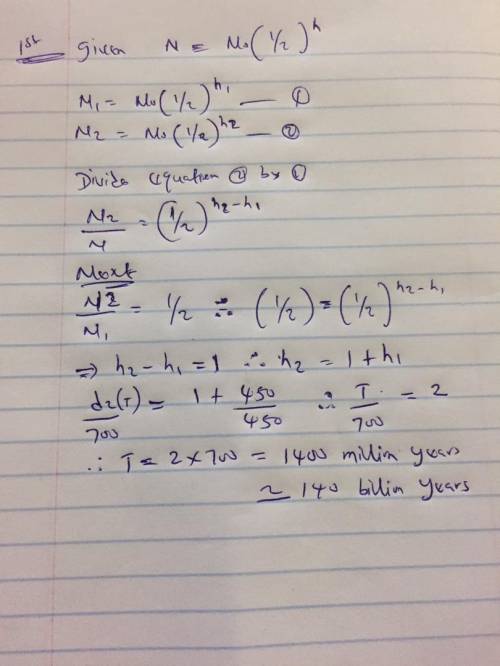Chemistry, 01.04.2021 15:30 jmadden513
Uranium, an important component of both nuclear weapons and nuclearreactors, has two major isotopes, U-238, which has a half-life of approximately billion years, and U-235, which has a half-life of approximately million years. Both were present in equal amounts at the time of thecreation of the Earth, billion years ago. How many years after the creationof the Earth had the amount of radiation from uranium decayed to half theamount present at the time of the creation of the Earth

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

You know the right answer?
Uranium, an important component of both nuclear weapons and nuclearreactors, has two major isotopes,...
Questions


SAT, 29.11.2021 20:10



Mathematics, 29.11.2021 20:10

Mathematics, 29.11.2021 20:10


English, 29.11.2021 20:10


Mathematics, 29.11.2021 20:10

History, 29.11.2021 20:10

English, 29.11.2021 20:10


Mathematics, 29.11.2021 20:10



Mathematics, 29.11.2021 20:10



Mathematics, 29.11.2021 20:10