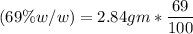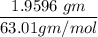Chemistry, 19.03.2021 18:10 rileybantaos1c8s
Mass of watch glass + filter paper = 105.98 g
Mass of watch glass + filter paper + crystallized product = 109.03 g
Mass of uncrystallized product (show work) =
Mass of methyl benzoate = 3.08 g
Volume of nitric acid used = 2.0 mL
Theoretical yield based on each of the starting materials
(Please use two Dimensional Analysis (DA) equations, one for the maximum amount of product obtainable from the amount of methyl benzoate you used and the other from the concentrated nitric acid, then use the lesser of the two to determine the Limiting Reagent; you must determine the number of moles in 2.00 mL of concentrated nitric acid [concentration 69.0% (w/w), and density (1.42 g/mL)].
Required:
a. Identity of the Limiting reagent (LR) based on the above two DA equations =
b. Max amount of product obtainable from the LR =
c. Mass of the product you obtained:

Answers: 2


Another question on Chemistry




Chemistry, 23.06.2019 18:20
Ahydrogen electron is elevated from level 1 to level 2. another electron is elevated from level 2 to level 4. the transition requiring the greatest energy change is?
Answers: 1
You know the right answer?
Mass of watch glass + filter paper = 105.98 g
Mass of watch glass + filter paper + crystallized pro...
Questions


Chemistry, 03.07.2019 17:40


Mathematics, 03.07.2019 17:40



Mathematics, 03.07.2019 17:40




Mathematics, 03.07.2019 17:40


Physics, 03.07.2019 17:40


English, 03.07.2019 17:40

Mathematics, 03.07.2019 17:40



Chemistry, 03.07.2019 17:40

Physics, 03.07.2019 17:40