
Chemistry, 18.03.2021 01:20 alondra6190
Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas, which is colorless.
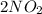 (g) ⇌
(g) ⇌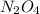 (g)
(g)
When the mixture was moved from room temperature to a higher temperature, the mixture turned darker brown. Which of the following conclusions about the mixture is true?
A. The forward reaction is exothermic.
B. The backward reaction has a negative net enthalpy.
C. The colorless gas has a higher enthalpy than the brown gas.
I know that is an exothermic reaction, but it shifts to left, so it should be a backward reaction, not a forward. Therefore, I don't think it is A. I am leaning towards B, but I would like someone to double-check my thinking. Please no fake answers or external links. Thanks in advance!

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Monkeys and bats have similar bone structure in their forelimbs. however, monkeys have longer forelimbs to use for climbing and swinging in trees. bats have shorter forelimbs to use for flight. which term best describes how monkey and bat forelimbs are related to each other? a. homologous b. embryonic c. analogous d. vestigial
Answers: 1

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3
You know the right answer?
Nitrogen dioxide gas is dark brown in color and remains in equilibrium with dinitrogen tetroxide gas...
Questions

Social Studies, 27.05.2020 02:03

Mathematics, 27.05.2020 02:03


















History, 27.05.2020 02:03



