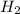Consider the following reaction:
Mg(s) + 2 HCl (aq) → H2(g) + MgCl2(aq)
If 3.65 mol of magnes...

Chemistry, 18.03.2021 01:00 carmencolon119
Consider the following reaction:
Mg(s) + 2 HCl (aq) → H2(g) + MgCl2(aq)
If 3.65 mol of magnesium and 3.65 mol of hydrochloric acid are reacted, how many
moles of hydrogen gas are produced?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

You know the right answer?
Questions


Computers and Technology, 19.02.2020 04:30

Computers and Technology, 19.02.2020 04:30


Biology, 19.02.2020 04:30


Mathematics, 19.02.2020 04:31


Mathematics, 19.02.2020 04:31











Mathematics, 19.02.2020 04:31

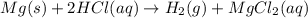
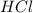 require = 1 mole of
require = 1 mole of 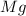
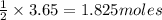 of
of