
Chemistry, 13.03.2021 04:50 genyjoannerubiera
Text 8.7 Using the virial equation of state for hydrogen at 298 K given in problem 7 (text 8.6), calculate a. The fugacity of hydrogen at 500 atm and 298 K b. The pressure at which they fugacity is twice the pressure c. The change in Gibbs free energy caused by a compression of 1 mole of hydrogen from 1 to 500 atm. What is the magnitude of the contribution to (c) caused by the non ideality of hydrogen

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
You know the right answer?
Text 8.7 Using the virial equation of state for hydrogen at 298 K given in problem 7 (text 8.6), cal...
Questions





English, 16.06.2020 22:57

Biology, 16.06.2020 22:57






Mathematics, 16.06.2020 22:57





Mathematics, 16.06.2020 22:57



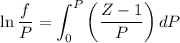
 ........(1)
........(1)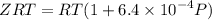
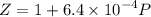
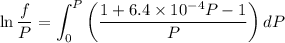
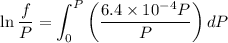
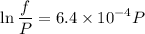
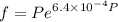
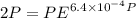
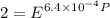
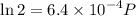
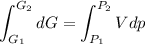
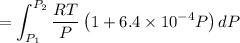
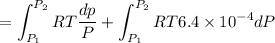
![$\Delta G=R[\ln\frac{P_2}{P_1}+6.4 \times 10^{-4}(P_2-P_1)]$](/tpl/images/1193/5881/be85b.png)
![$\Delta G=8.314\times 298[\ln\frac{500}{1}+6.4 \times 10^{-4}(500-1)]$](/tpl/images/1193/5881/16a41.png)


