
Chemistry, 13.03.2021 01:00 mvtthewisdead
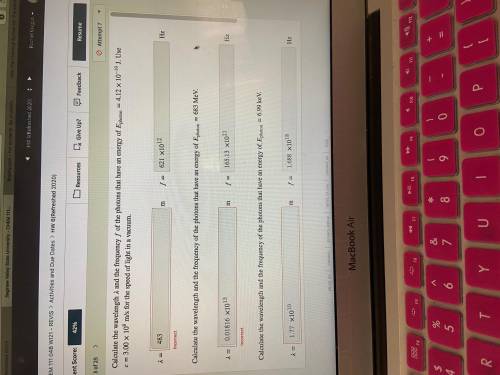
Calculate the wavelength and the frequency of the photons that have an energy of photon=4.12×10−19 J. Use =3.00×108 m/s for the speed of light in a vacuum.
Calculate the wavelength and the frequency of the photons that have an energy of photon=683 MeV.
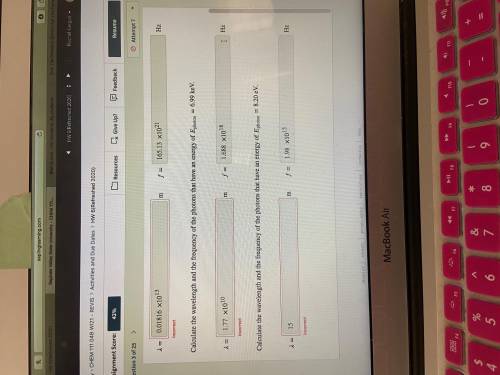
Calculate the wavelength and the frequency of the photons that have an energy of photon=6.99 keV.
Calculate the wavelength and the frequency of the photons that have an energy of photon=8.20 eV.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

You know the right answer?
Calculate the wavelength and the frequency of the photons that have an energy of photon=4.12×10−19 J...
Questions









Chemistry, 17.06.2021 22:20


Mathematics, 17.06.2021 22:20

Mathematics, 17.06.2021 22:20

Mathematics, 17.06.2021 22:20


English, 17.06.2021 22:20

Mathematics, 17.06.2021 22:20






