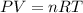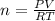Chemistry, 10.03.2021 09:30 heavenwagner
If the pressure, volume, and tempature of a gas are known which can most likely be found by using the ideal gas law
• the molar amount of the gas
•the partial pressure of the gas
•the stand temperature
•the molar mass

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2
You know the right answer?
If the pressure, volume, and tempature of a gas are known which can most likely be found by using th...
Questions



Biology, 09.03.2020 04:31



Mathematics, 09.03.2020 04:31

English, 09.03.2020 04:31


Mathematics, 09.03.2020 04:33

Mathematics, 09.03.2020 04:33



Biology, 09.03.2020 04:34

Physics, 09.03.2020 04:35

Mathematics, 09.03.2020 04:36

English, 09.03.2020 04:36

Mathematics, 09.03.2020 04:36


World Languages, 09.03.2020 04:36

English, 09.03.2020 04:36