
1. Place the five substances in order of increasing pH — from most acidic to most basic. (2 points) Click here to enter text. 2. Orange juice has a pH of 4. What color would you expect the paper test strip to be after it is dipped in a sample of orange juice? (2 points) Click here to enter text. 3. Determining the pH of substances such as purple grape juice and catsup using test strips can be difficult. Why? (3 points) Click here to enter text 4. Tear-free shampoos are advertised as having a pH similar to that of human tears. What pH would you expect tear-free shampoos to be? Use evidence from the lab activity to support your response. (5 points) Click here to enter text. 5. What is the approximate hydronium ion concentration and hydroxide ion concentration in a cup of tea? Which is higher? (Refer back to the chart of pH in the lesson if you need to.) (4 points) Click here to enter text. 6. What is the approximate hydronium ion concentration and hydroxide ion concentration in bleach? Which is higher? (4 points) Click here to enter text. 7 plzz help mee!
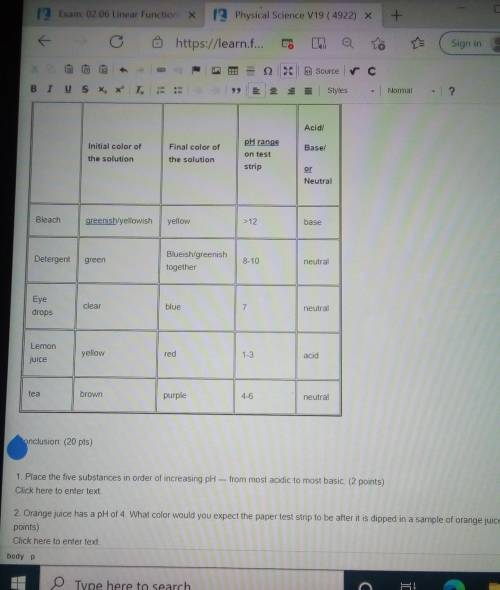

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3
You know the right answer?
1. Place the five substances in order of increasing pH — from most acidic to most basic. (2 points)...
Questions

History, 31.01.2020 23:02


History, 31.01.2020 23:02



Social Studies, 31.01.2020 23:03

History, 31.01.2020 23:03


English, 31.01.2020 23:03

English, 31.01.2020 23:03


Biology, 31.01.2020 23:03



Mathematics, 31.01.2020 23:03


Social Studies, 31.01.2020 23:03

Social Studies, 31.01.2020 23:03

Mathematics, 31.01.2020 23:03





