Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3



Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
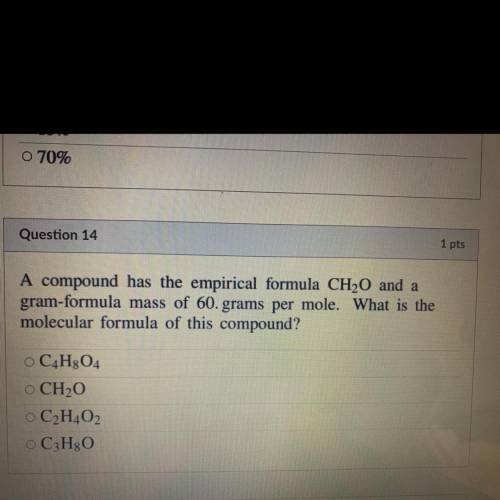
A compound has the empirical formula CH20 and a
gram-formula mass of 60. grams per mole. What is th...
Questions


History, 10.07.2019 13:00


Mathematics, 10.07.2019 13:00


Social Studies, 10.07.2019 13:00


History, 10.07.2019 13:00


Social Studies, 10.07.2019 13:00