
Chemistry, 28.02.2021 21:10 cicimarie2018
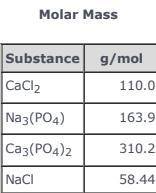
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s)+ 6NaCl(aq) Use the balanced equation and the Molar Mass table above to answer the following question. Suppose 163.9 g of Na3(PO4) in solution mixed with sufficient CaCl2 in solution yields 116 g ofCa3(PO4)2(s). What is the percent yield of Ca3(PO4)2(s)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s)+ 6NaCl(aq) Use the balanced equation and the Molar Mass ta...
Questions



Biology, 07.09.2019 16:10

Biology, 07.09.2019 16:10

Biology, 07.09.2019 16:20








Physics, 07.09.2019 16:20

Physics, 07.09.2019 16:20









