
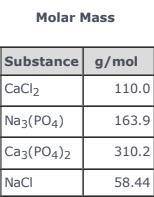
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s)+ 6NaCl(aq) Use the balanced equation and the Molar Mass table above to answer the following question. How much Ca3(PO4)2(s) could be produced in an industrial process if 55.00 g of CaCl2 in solution reacted completely with sufficient Na3(PO4)(aq)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s)+ 6NaCl(aq) Use the balanced equation and the Molar Mass ta...
Questions

History, 24.08.2019 11:00

English, 24.08.2019 11:00

Computers and Technology, 24.08.2019 11:00


Biology, 24.08.2019 11:00


Mathematics, 24.08.2019 11:00

Mathematics, 24.08.2019 11:00

Chemistry, 24.08.2019 11:00

Mathematics, 24.08.2019 11:00


Mathematics, 24.08.2019 11:00


Biology, 24.08.2019 11:10


Mathematics, 24.08.2019 11:10

History, 24.08.2019 11:10





