
Chemistry, 28.02.2021 17:20 dragador7601
When some solid ammonium nitrate was dissolved in water the temperature decreased from 22 oC to 3 oC. What can be deduced from this observation?
The dissolving is endothermic and ∆H is positive. The dissolving is endothermic and ∆H is negative. The dissolving is exothermic and ∆H is positive. The dissolving is exothermic and ∆H is negative.
Which is a correct statement about an endothermic reaction?
A. The bonds in the reactants are stronger than in the products and ∆H is positive.
B. The bonds in the products are stronger than in the reactants and ∆H is positive.
C. The bonds in the reactants are stronger than in the products and ∆H is negative
D. The bonds in the products are stronger than in the reactants and ∆H is negative.
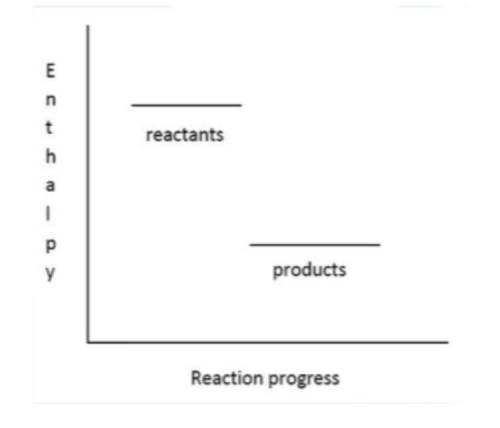
Which is a correct statement about the following() enthalpy level diagram of a reaction? A. The reaction is exothermic and △H is positive.
E. The reaction is exothermic and △H is negative.
F. The reaction is endothermic and △H is positive.
G. The reaction is endothermic and △H is negative.
Which statements are correct for all exothermic reactions?
I. The products are more stable than the reactants.
II. The bonds in the products are stronger than the bonds in the reactants
III. The enthalpy of the products is less than the enthalpy of the reactants
A. I and II only
B. I and III only
C. II and III only
D. I, II and III

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 04:00
Actual ingredients of lab (the cookies i am actually making) 1/2 cup sugar 1/2 cup brown sugar 1 1/3 stick margarine 1 egg 1/2 tsp salt 1 tsp vanilla 1/2 tsp baking soda 1 1/2 cup flour 1 1/3 cup chocolate chip can you answer the questions below ? discussion 1. suppose you are given the following amounts of ingredients: 1 dozen eggs 24 tsp. of vanilla 1 lb. (82 tsp.) of salt 1 lb. (84 tsp.) of baking soda 3 cups of chocolate chips 5 lb. (11 cups) of sugar 2 lb. (4 cups) of brown sugar 1 lb. (4 sticks) of margarine a. for each ingredient, calculate how many cookies could be prepared if all of that ingredient were consumed. (for example, the recipe shows that using 1 egg- with the right amounts of the other ingredients- yields 24 cookies. how many cookies can you make if the recipe is increased proportionately for 12 eggs? ) b. to determine the limiting reactant for the new ingredients list, identify which ingredient will result in the fewest number of cookies. c. what is the maximum number of cookies that can be produced from the new amounts of ingredients?
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
When some solid ammonium nitrate was dissolved in water the temperature decreased from 22 oC to 3 oC...
Questions

Mathematics, 26.06.2019 01:00

Mathematics, 26.06.2019 01:00

Mathematics, 26.06.2019 01:00

Health, 26.06.2019 01:00

Social Studies, 26.06.2019 01:00


Mathematics, 26.06.2019 01:00

History, 26.06.2019 01:00



Mathematics, 26.06.2019 01:00

History, 26.06.2019 01:00

History, 26.06.2019 01:00

Mathematics, 26.06.2019 01:00

English, 26.06.2019 01:00

Mathematics, 26.06.2019 01:00






