Chemistry, 28.02.2021 17:20 lailabirdiemae
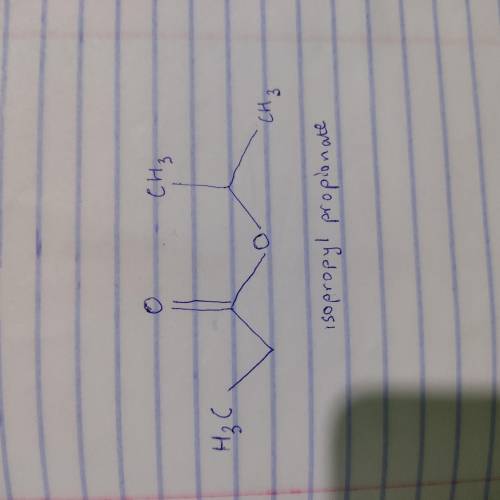
An unknown compound X has the empirical formula C3H6O and a molecular ion peak in its mass spectrum at m/z 116. X shows no IR absorption at 3200-3600 cm^-1 but shows a peak at 1740 cm^-1. The H NMR spectral data of X is shown below. What is the most likely structure of X? 22. An unknown compound X has the molecular formula C6H14O. X shows a strong peak in its IR spectrum at 3000 cm^-1. The 1H NMR spectral data of N are given below. What is the most likely structure of X?
Absorption ζ H ratio
triplet 1.0 3
double 1.0 6
quartet 2.0 2
septet 3.5 1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1


Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
An unknown compound X has the empirical formula C3H6O and a molecular ion peak in its mass spectrum...
Questions

English, 11.02.2020 17:13


Computers and Technology, 11.02.2020 17:13

Mathematics, 11.02.2020 17:13









English, 11.02.2020 17:14