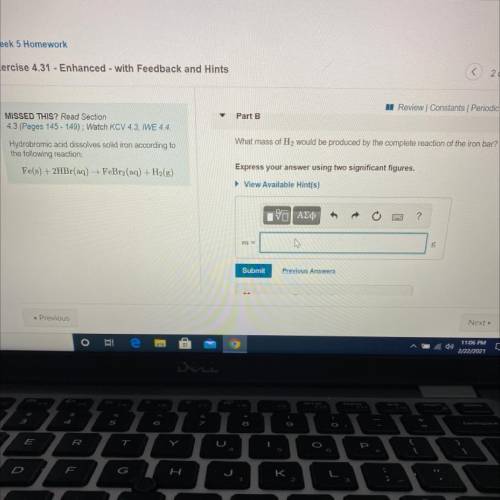
Hydrobromic acid dissolves solid iron according to
the following reaction:
Fe(s) + 2HBr(aq) →...

Chemistry, 23.02.2021 09:10 suttonfae336
Hydrobromic acid dissolves solid iron according to
the following reaction:
Fe(s) + 2HBr(aq) → FeBr2(aq) + H2(g)
What mass of H2 would be produced by the complete reaction of the iron bar?
Express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3


Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
Questions



Mathematics, 21.05.2021 18:20

Mathematics, 21.05.2021 18:20

History, 21.05.2021 18:20



Arts, 21.05.2021 18:20

Mathematics, 21.05.2021 18:20



Mathematics, 21.05.2021 18:20

Mathematics, 21.05.2021 18:20

Social Studies, 21.05.2021 18:20

Mathematics, 21.05.2021 18:20


Mathematics, 21.05.2021 18:20

Mathematics, 21.05.2021 18:20


Mathematics, 21.05.2021 18:20



