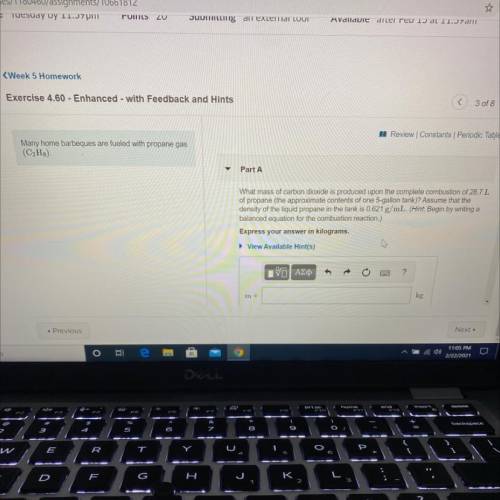
Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is...

Chemistry, 23.02.2021 09:20 keilajohnson810
Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is produced upon the complete combustion of 28.7L
of propane (the approximate contents of one 5-gallon tank)? Assume that the
density of the liquid propane in the tank is 0.621 g/mL.
Express your answer in kilograms.
Will give brainliest!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

You know the right answer?
Questions

Mathematics, 07.01.2021 19:50

Mathematics, 07.01.2021 19:50

Mathematics, 07.01.2021 19:50

Computers and Technology, 07.01.2021 19:50


History, 07.01.2021 19:50

English, 07.01.2021 19:50


Social Studies, 07.01.2021 19:50




Advanced Placement (AP), 07.01.2021 19:50

Mathematics, 07.01.2021 19:50

Mathematics, 07.01.2021 19:50

Social Studies, 07.01.2021 19:50

Mathematics, 07.01.2021 19:50

History, 07.01.2021 19:50

Social Studies, 07.01.2021 19:50

English, 07.01.2021 19:50



