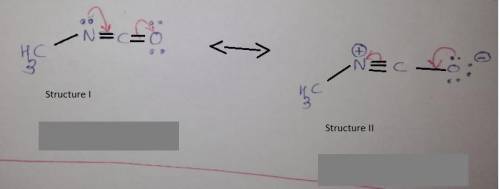
Methyl isocyanate, shown as resonance structure 1, can also be represented by other resonance structures. Draw the next most important resonance contributor. Then add curved arrows to each structure to show delocalization of electron pairs to form the other structure. Include lone pairs of electrons, formal charges, and hydrogen atoms. You can add condensed hydrogens using the More menu, selecting +H and clicking on the carbon as many times as needed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Methyl isocyanate, shown as resonance structure 1, can also be represented by other resonance struct...
Questions


Mathematics, 30.11.2019 08:31




History, 30.11.2019 08:31





Chemistry, 30.11.2019 08:31

English, 30.11.2019 08:31




Health, 30.11.2019 08:31

Geography, 30.11.2019 08:31

Mathematics, 30.11.2019 08:31

Biology, 30.11.2019 08:31

 - bonds = 0
- bonds = 0