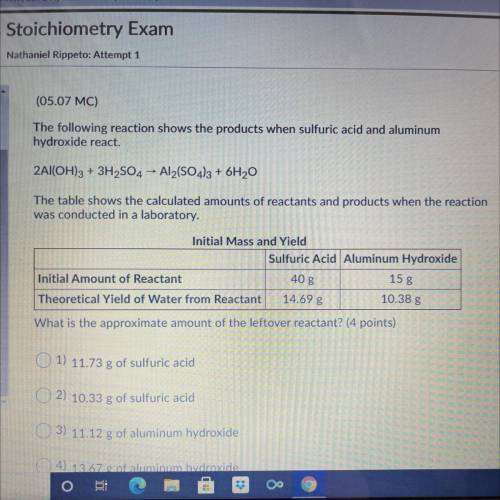
The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
2A...

The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
2Al(OH)3 + 3H2SO4 - Al2(SO4)3 + 6H20
The table shows the calculated amounts of reactants and products when the reaction
was conducted in a laboratory.
What is the approximate amount of the leftover reactant?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2


Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
Questions


Advanced Placement (AP), 15.12.2020 16:40

History, 15.12.2020 16:40





Mathematics, 15.12.2020 16:40



Mathematics, 15.12.2020 16:40

English, 15.12.2020 16:40










