Fill in the blanks
A metallic bond is the
attraction between the valence electrons and the po...

Chemistry, 12.02.2021 09:20 brownw2005
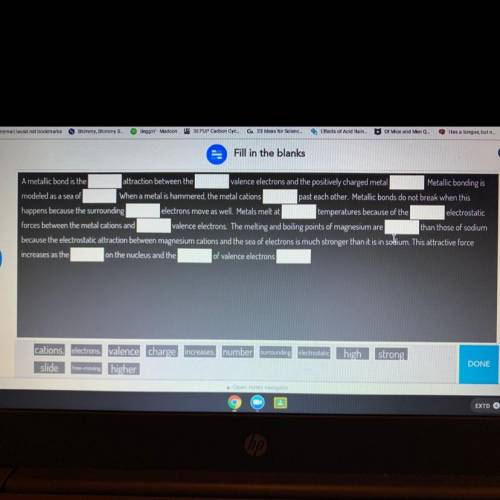
Fill in the blanks
A metallic bond is the
attraction between the valence electrons and the positively charged metal
Metallic bonding is
modeled as a sea of
When a metal is hammered, the metal cations past each other. Metallic bonds do not break when this
happens because the surrounding electrons move as well. Metals melt at temperatures because of the electrostatic
forces between the metal cations and valence electrons. The melting and boiling points of magnesium are than those of sodium
because the electrostatic attraction between magnesium cations and the sea of electrons is much stronger than it is in sodium. This attractive force
increases as the on the nucleus and the of valence electrons
Surrounding
electrostatic
high
strong
cations. electrons valence charge increases. number
slide free-moving higher
DONE
Open notes navigator

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

You know the right answer?
Questions

Chemistry, 24.05.2021 19:10



Mathematics, 24.05.2021 19:10


English, 24.05.2021 19:10




Mathematics, 24.05.2021 19:10

Mathematics, 24.05.2021 19:10


History, 24.05.2021 19:10




English, 24.05.2021 19:10



Mathematics, 24.05.2021 19:10



