
Chemistry, 09.02.2021 01:50 courtney3652
The equilibrium constant, Kc, for the following reaction is 83.3 at 500 K. PCl3(g) Cl2(g) PCl5(g) Calculate the equilibrium concentrations of reactant and products when 0.453 moles of PCl3 and 0.453 moles of Cl2 are introduced into a 1.00 L vessel at 500 K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1


Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

You know the right answer?
The equilibrium constant, Kc, for the following reaction is 83.3 at 500 K. PCl3(g) Cl2(g) PCl5(g) Ca...
Questions


History, 27.12.2019 10:31


Mathematics, 27.12.2019 10:31

Mathematics, 27.12.2019 10:31

Biology, 27.12.2019 10:31



Computers and Technology, 27.12.2019 10:31

Mathematics, 27.12.2019 10:31

History, 27.12.2019 10:31


Mathematics, 27.12.2019 10:31

Mathematics, 27.12.2019 10:31



Social Studies, 27.12.2019 10:31


Mathematics, 27.12.2019 10:31

Mathematics, 27.12.2019 10:31

![[PCl_3]=[Cl_2]=0.068M](/tpl/images/1103/3296/1ad55.png)
![[PCl_5]=0.385M](/tpl/images/1103/3296/9d9e6.png)
![Kc=\frac{[PCl_5]}{[Cl_2][PCl_3]}](/tpl/images/1103/3296/6b2bc.png)
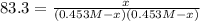
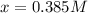
![[PCl_3]=[Cl_2]=0.453M-0.385M=0.068M](/tpl/images/1103/3296/82c25.png)


