
Chemistry, 30.01.2021 05:40 hdjsjfjruejchhehd
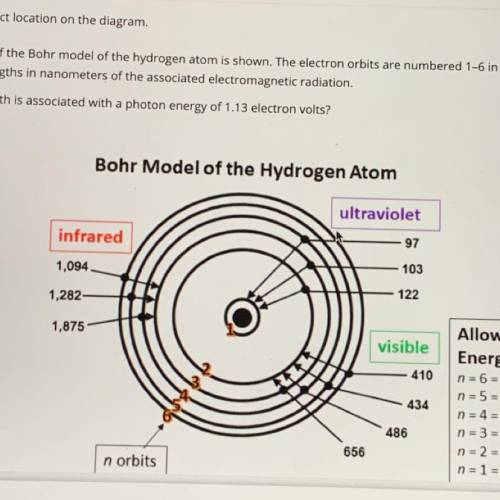
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-6 in orange and black. The black numbers are the wavelengths in nanometers of the associated electromagnetic radiation. Which wavelength is associated with a photon energy of 1.13 electron volts?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-...
Questions







Biology, 28.03.2020 00:47


Mathematics, 28.03.2020 00:47

History, 28.03.2020 00:48



History, 28.03.2020 00:48

Mathematics, 28.03.2020 00:49


Computers and Technology, 28.03.2020 00:52




English, 28.03.2020 00:52



