
Chemistry, 30.01.2021 05:40 dominguezjose625
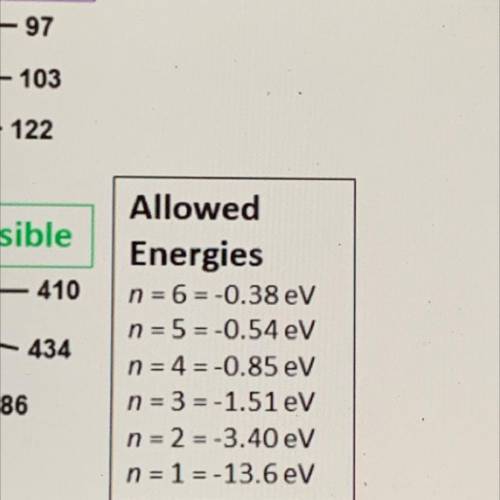
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-6 in orange and black. The black numbers
are the wavelengths in nanometers of the associated electromagnetic radiation.
Which wavelength is associated with a photon energy of 1.13 electron volts?
Bohr Model of the Hydrogen Atom
ultraviolet
infrared
-97
103
1,094
122
1,282
1,875
visible
410
434
Allowed
Energies
n = 6-0.38 eV
n = 5=-0.54 eV
n = 4 = -0.85 eV
n = 3 = -1.51 eV
n = 2 = -3.40 eV
n = 1 = -13.6 eV
486
656
In orbits
n orbits

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1
You know the right answer?
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-...
Questions

Mathematics, 12.05.2021 19:50



Mathematics, 12.05.2021 19:50

Social Studies, 12.05.2021 19:50

Mathematics, 12.05.2021 19:50


Mathematics, 12.05.2021 19:50


History, 12.05.2021 19:50

Arts, 12.05.2021 19:50

Mathematics, 12.05.2021 19:50

Mathematics, 12.05.2021 19:50






Social Studies, 12.05.2021 19:50

Mathematics, 12.05.2021 19:50



